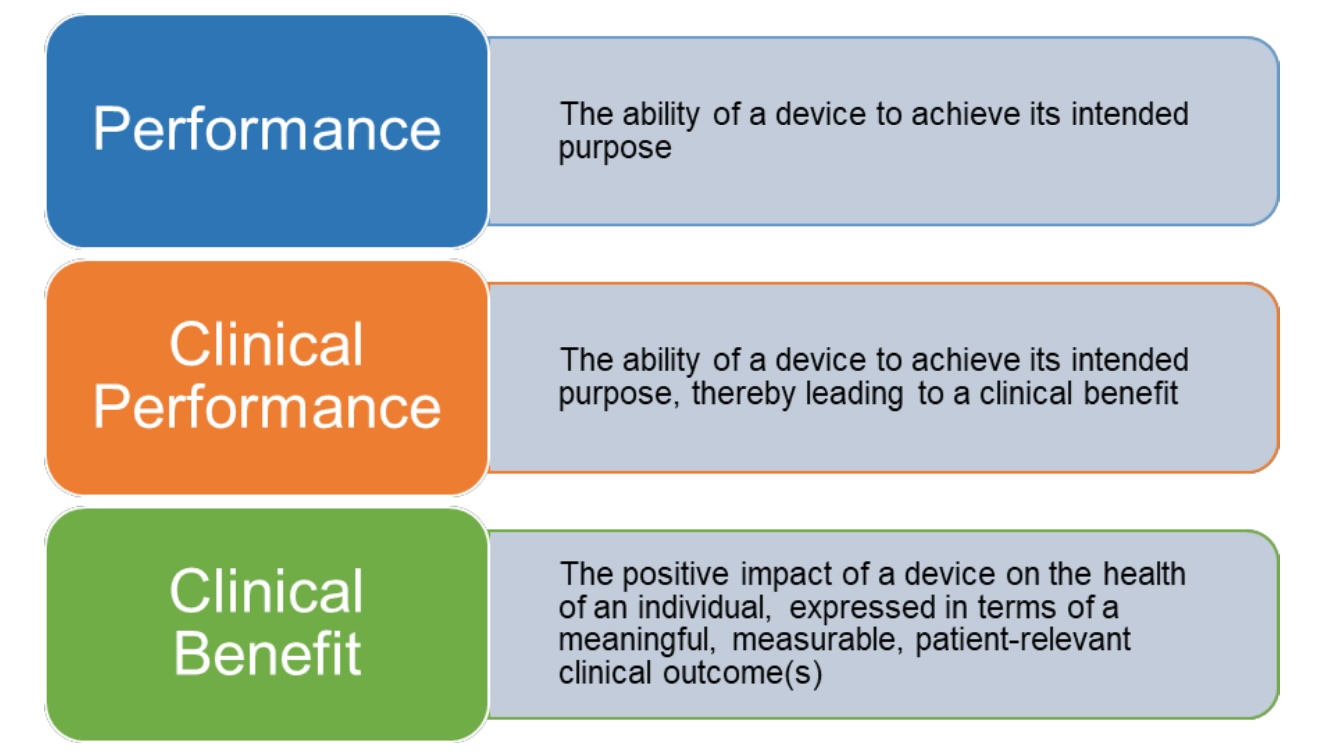
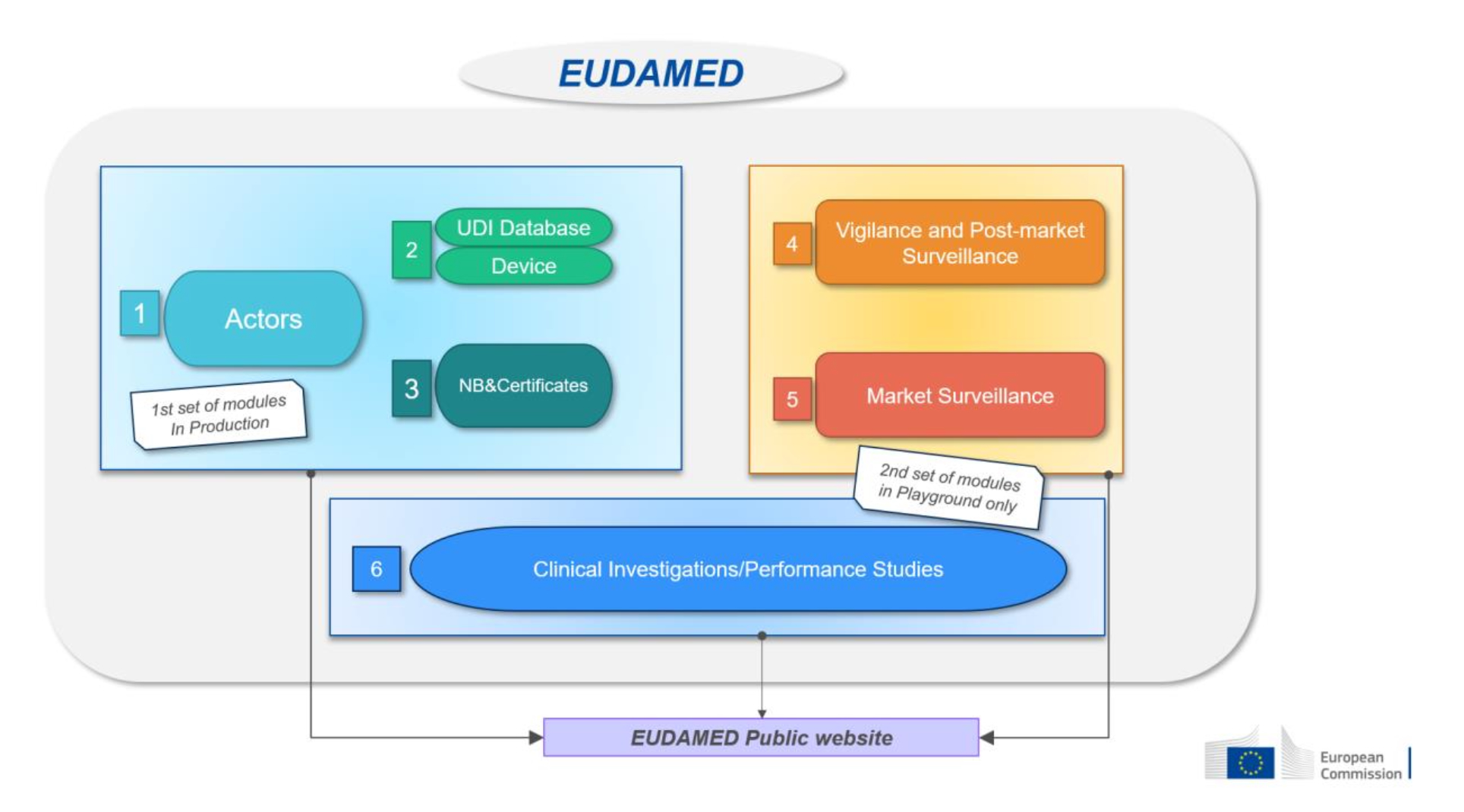
Devices without an intended medical purpose: how to comply with the EU MDR legal requirements?
EU medical device regulation (MDR) 2017/745 Annex XVI products are those with only an aesthetic or another non-medical purpose claim, but which are similar to medical devices in terms of functioning and risk profile.
Read more