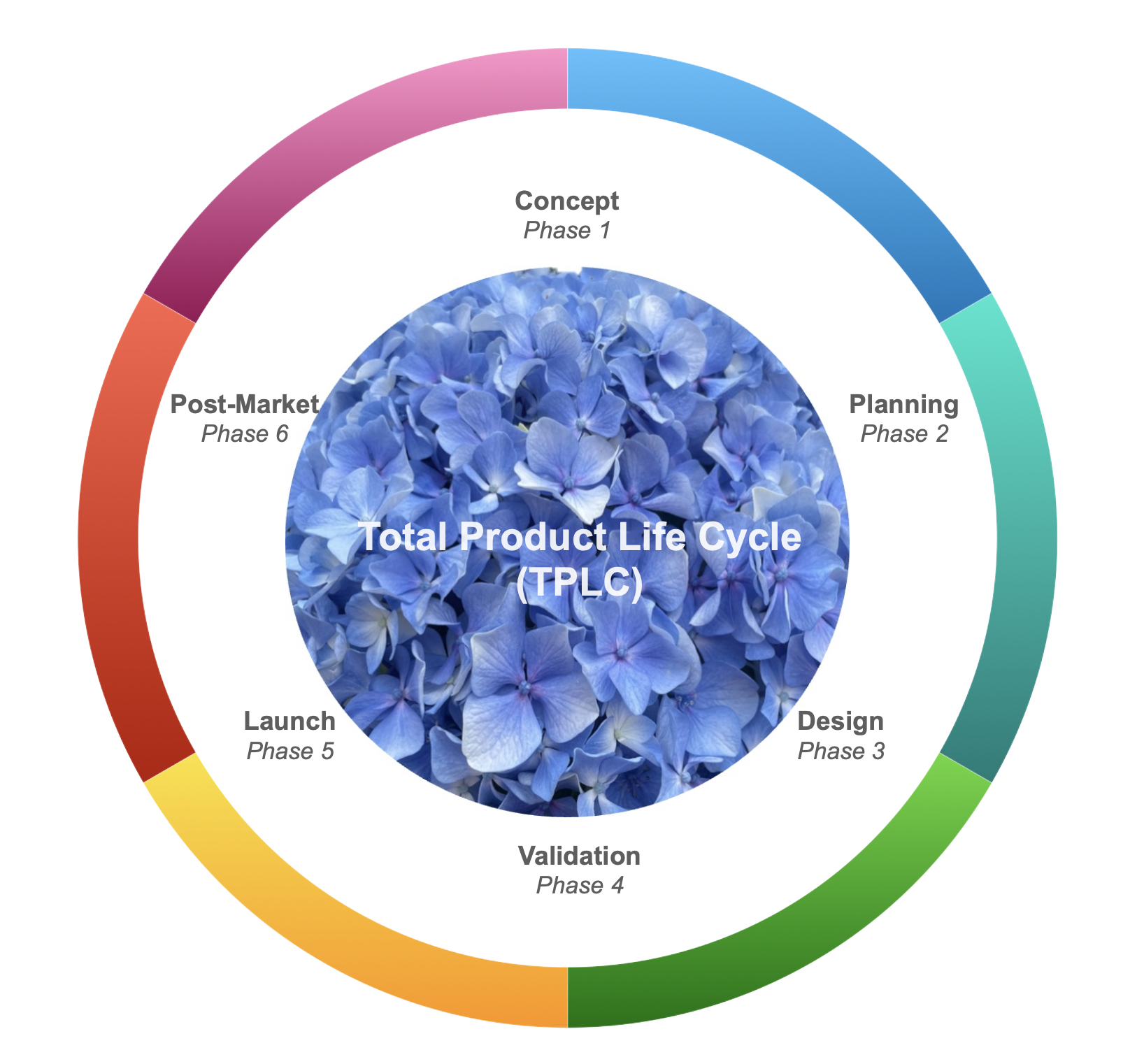
Total product life cycle: grasp your competitive advantage
In the complex and ever-changing medical device industry, personalised support from experienced, professional and well qualified technical specialists is critical. As such, our mission is to ensure patient safety, while supporting manufacturers with timely access to global markets. We understand the challenges medical devices manufacturers face to bring compliant products to the market efficiently and safely; and with that in mind, we offer a range of flexible product review and writing services with an eye on providing efficient pathways to bring new medical products to patients in need, based on a Total Product Life Cycle (TPLC).
An understanding of the complex clinical and regulatory requirements early in the product lifecycle makes the difference in gaining the competitive advantage needed to bring a medical product to the market. For that, it is essential a strategic clinical and regulatory planning to maximise resources and reduce the risk of costly re-developments later in the product lifecycle.
- In the Concept Phase 1, an independent analysis will determine whether your product qualifies as a medical device, its intended use, the initial risk analysis, potential markets and routes, and the draft of regulatory documents necessary for compliance in different global markets.- In the Planning Phase 2, design inputs are further defined based on the customer needs and technical requirements, with a thorough concept development, prototype analysis, initial testing, design verification, risk analysis, and user feedback. It's time to think about the regulatory strategy to different regions of the globe, according to the market needs.
- In the Design Phase 3, the development of manufacturing processes, verification and validation of the medical product enter into full force. It's time to set a clinical strategy that endorses the product claims. Retrospective and/or prospective clinical trials need to be defined to assess the efficacy and safety of the device in terms of regulatory requirements.
- In the Validation Phase 4, the final validation of the manufacturing process occurs and there is the need to prepare the introduction of the product to the market. Market plans and forecasts need to be drawn, together with the clinical validation data for final labelling and regulatory submission.
- In the Launch Phase 5, regulatory approval and certification launch the medical device into the market - hurray!
But the work is not over...
- Post-market Phase 6 begins. It's time for post-market surveillance (PMS), and post-market clinical follow-up (PMCF) studies to close the gaps on any risks, complains and adverse events. Based on these data, new designs, new indications for use, new ideas will develop and a new cycle of product development begins.
Product improvements are a constant need to drive any field further. Only by renovating can a medical device face the new technologies that are constantly coming to the market, and be able to remain State-of-The-Art (SoTA).
Just like nature drives a constant rebirth, also medical products need a constant feed not to decline and become redundant. Total Product Life Cycle (TPLC) is a way of not flattening the sale curve, and to plan future expansion. It also prevents trying too many things at once, particularly as a Start-Up/SME. It also helps to prioritise rationally based on Real-World Evidence (RWE) and true input of technical data.
TPLC is the only way for medical device development and growth. It will keep you on a straight path of continued success.
Let us help you bloom your medical device using a TPLC approach...
Catarina Carrão
by Catarina Carrão
(aka Ana Catarina Ribeiro Carrão)
