EUDAMED: speed-up
On the 23rd of January 2024, the European Commission released a proposal with aims to launch parts of EUDAMED that are already finalised earlier than previously thought. There will be a gradual roll out of all six modules between Q4/2024 and 2026, with mandatory use delayed untill there’s confirmation on full functionality (2027-2029).
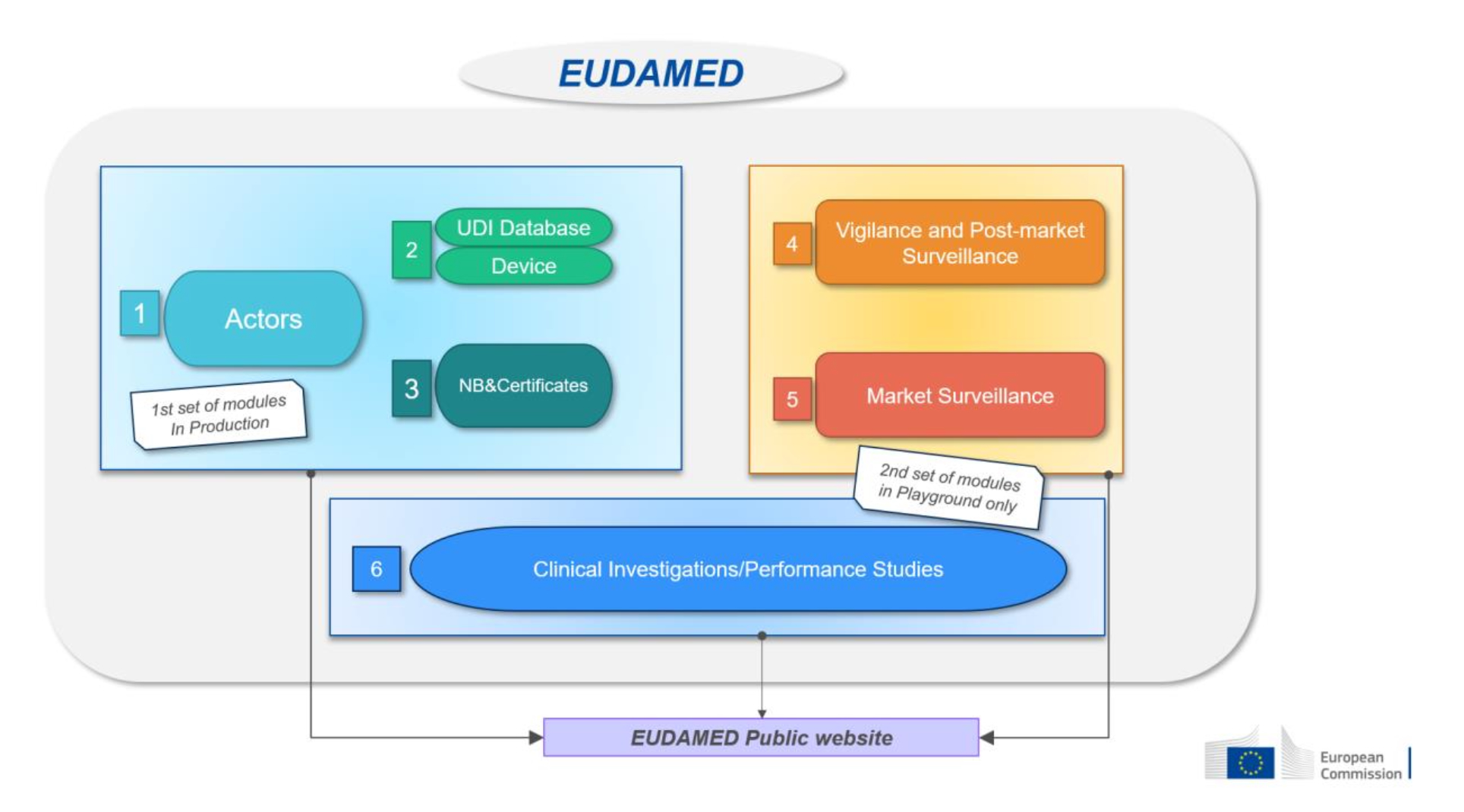
Three Eudamed modules have been available for voluntary use since December 2020 (Actors) and October 2021 (UDI/Devices; Notified bodies/Certificates). Two further modules (Market Surveillance; Post-Market Surveillance and Vigilance) are
expected to be completed in Q2/2024. The last module (Clinical investigations/Performance studies) will not be completed before Q3/2026.
Pursuant to the current MDR rules, Eudamed can only be used mandatorily from a certain date after the Commission has verified that Eudamed is fully functional and has published a notice to that effect. Therefore, the delayed development of the last module holds back the mandatory use of the electronic systems that have been completed already. The mandatory use of all six modules thus cannot be expected before Q4/2027, with additional transitional periods not ending before Q2/2029.
The mandatory use of the European database on medical devices, EUDAMED, is key for the effective and efficient implementation of the Medical Device and IVD Regulations. It will increase transparency in the EU, providing an overview of all medical devices available on the European market.
Links:
https://ec.europa.eu/commission/presscorner/detail/en/IP_24_346
https://health.ec.europa.eu/system/files/2024-01/mdr_in-vitro-proposal.PDF
Text & image: Ana Catarina Ribeiro Carrão
Three Eudamed modules have been available for voluntary use since December 2020 (Actors) and October 2021 (UDI/Devices; Notified bodies/Certificates). Two further modules (Market Surveillance; Post-Market Surveillance and Vigilance) are
expected to be completed in Q2/2024. The last module (Clinical investigations/Performance studies) will not be completed before Q3/2026.
Pursuant to the current MDR rules, Eudamed can only be used mandatorily from a certain date after the Commission has verified that Eudamed is fully functional and has published a notice to that effect. Therefore, the delayed development of the last module holds back the mandatory use of the electronic systems that have been completed already. The mandatory use of all six modules thus cannot be expected before Q4/2027, with additional transitional periods not ending before Q2/2029.
The mandatory use of the European database on medical devices, EUDAMED, is key for the effective and efficient implementation of the Medical Device and IVD Regulations. It will increase transparency in the EU, providing an overview of all medical devices available on the European market.
Links:
https://ec.europa.eu/commission/presscorner/detail/en/IP_24_346
https://health.ec.europa.eu/system/files/2024-01/mdr_in-vitro-proposal.PDF
Text & image: Ana Catarina Ribeiro Carrão